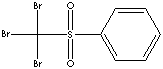
PRODUCT IDENTIFICATION
CLASSIFICATION
photosensitive material
EXTRA NOTES
PHYSICAL AND CHEMICAL PROPERTIES
REFRACTIVE INDEX
EXTERNAL LINKS & GENERAL DESCRIPTION
Wikipedia Linking - Photoinitiator
PubChem Compound Summary - Tribromomethyl phenyl sulfone
http://www.ebi.ac.uk/chebi/ - Tribromomethyl phenyl sulfone
http://www.ncbi.nlm.nih.gov/ - Tribromomethyl phenyl sulfone
Material Safety Data Sheet
http://www.beilstein-journals.org/
A halogenmethylsulfonyl moiety is incorporated in numerous active herbicides
and fungicides. The synthesis of tribromomethyl phenyl sulfone derivatives as
novel potential pesticides is reported. The title sulfone was obtained by
following three different synthetic routes, starting from 4-chlorothiophenol or
4-halogenphenyl methyl sulfone. Products of its subsequent nitration were
subjected to the SNAr reactions with ammonia, amines, hydrazines and
phenolates to give 2-nitroaniline, 2-nitrophenylhydrazine and diphenyl ether
derivatives. Reduction of the nitro group of
4-tribromomethylsulfonyl-2-nitroaniline yielded the corresponding
o-phenylenediamine substrate for preparation of structurally varied
benzimidazoles.
Local:
Polymerization is initiated generally by free radical. There are four types of
free radical initiators which initiate polymerization.
- Peroxides including hydroperoxides (tertiary-butyl hydroperoxide, benzoylperoxide)
- Azocompound thermal initiators (azoisobutyronitrile)
- Redoxinitiators (mixture of iron(III) acetylacetonate): free radicals are formed by one-electron transferreactions. Useful in low temperature and emulsion polymerization
- Photoinitiators (benzoin, benzil dimethylketal)
The main advantage of polymerization started by photoinitiators is temperature-independence and easy control. It can be conducted at very low temperatures and can be stopped simply by removing the light source. Photoinitiators are compounds that break down into free radicals upon exposure to ultraviolet radiation. Photoinitiators undergo a unimolecular bond cleavage upon irradiation to yield free radicals (benzoin esters; benzil ketals; alpha-dialkoxy acetophenones; alpha-hydroxy-alkylphenones; alpha-amino alkyl- phosphine; acylphosphine oxides). Another type of photoinitiators undergo a bimolecular reaction where the excited state of the photoinitiator interacts with a second molecule (a coinitiator) to generate free radicals(benzo phenones,amines; thioxanthones,amines; titanocenes). Photoinitiators are widely applied in UV curing inks, wood coatings, paper coatings, optical fiber, PCB, screen printing , paper varnish and other surface coatings.
The common examples of sulfone compounds are dapsone (4,4'-sulfonylbisbenzenamine) and its derivative. Dapsone (4,4'-sulfonylbisbenzenamine) is a parent compound in preparing sulfonamide antibacterial agents such as acedapsone, acetosulfone, glucosulfone, sulfoxone and solapsone. Dapsone and its derivatives have bacteriostatic activity against a broad spectrum of gram-negative and gram-positive organisms. Dapsone is a primary treatment for Dermatitis herpetiformis and in the treatment of leprosy (Mycobacterium leprae infections) leads to apparent improvementas. It is used in the prophylaxis of falciparum malaria. It is a white crystalline powder; melting point 175 - 180C; insoluble in water and vegetable oils. Dapsone is also widely used in polymer industry as a cross linkage agent in manufacturing polysulfone amide engineering plastics. Sulfone-containing engineering plastics are thermally stable and have high resistance property to acids and alkalis. Tazobactam is a penicillanic acid sulfone derivative which acts as a beta-lactamase inhibitor similar to sulbactam. Tazobactam is used in combination with piperacillin to widen the spectrum of activity. Sulfone class compounds which have a sulfonyl group attached to two carbon atoms are active as an antibiotic.
Tribromomethyl phenyl sulfone is used as a photoinitiator for UV-curable systems, inks and varnishes. It is used as an intermediate for the synthesis of pharmaceuticals and other organic compounds.
APPEARANCE
ASSAY
BROMINE CONTENT
ca 60%
HAZARD OVERVIEW
GHS
PICTOGRAMS

HAZARD STATEMENTS
H319 Causes serious eye irritation
H317 May cause
an allergic skin reaction
PRECAUTIONARY STATEMENTS
P310 Immediately call a POISON CENTER or doctor/physician
P280
Wear eye protection/face protection
P501 Dispose of contents/container through a waste management company authorized
by the local government.
![]() Xi
Irritant
Xi
Irritant
RISK PHRASES
SAFETY PHRASES
26 In case of contact with eyes, rinse immediately with
plenty of water and seek medical advice
37/39 Wear suitable gloves and eye/face protection
PRICE INFORMATION